A new wood-based material that conducts ions 10–100 times better than other polymers could find use in next-generation solid-state lithium-ion batteries. The material, which was fabricated by researchers at Brown University and the University of Maryland in the US, combines copper and cellulose nanofibrils and could be used as either a solid battery electrolyte or as an ion-conducting binder for the cathode of an all-solid-state battery.
Lithium-ion batteries are widely employed in applications ranging from mobile phones to electric vehicles. These devices have a high capacity and high energy density, which means they can store a lot of charge very quickly. During charging, lithium ions move from the cathode to the anode though an electrolyte, which is usually made from a lithium salt dissolved in a liquid organic solvent. While this type of electrolyte works well, at high currents, needle-like lithium-metal structures called dendrites form on the anode surface and flow into the electrolyte. These unwanted structures eventually pierce the barrier separating the anode and cathode, causing the battery to short or even, in some cases, to ignite.
Polymer ion conductor
To overcome this problem, researchers are looking to replace the liquid electrolyte in these devices with a solid-state one that is harder for the dendrites to grow through. Most solid electrolyte materials studied to date are based on ceramics, which make good ionic conductors but are also rigid and brittle. This makes it difficult to integrate them into electrodes, and they are also prone to cracking or breaking during repeated battery charging and discharging.
Polymer ion conductors do not suffer from these drawbacks, but they also do not conduct ions as well as ceramics – or at least, they didn’t until researchers led by Liangbing Hu of Maryland’s Department of Materials Science and Engineering and Yue Qi of Brown’s School of Engineering developed their solution.
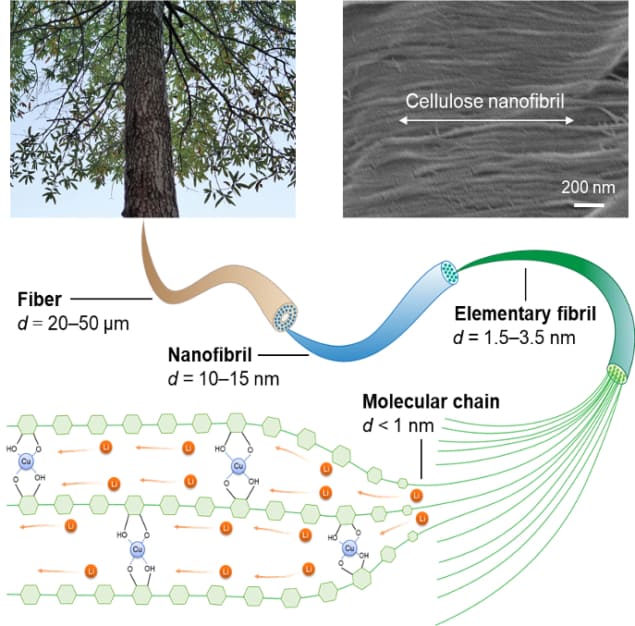
The new material made by Hu, Qi and colleagues is based on copper-containing cellulose nanofibrils, which are polymer tubes derived from wood. This combination allows the normally ion-insulating cellulose to rapidly transport lithium ions along the direction of the polymer chains thanks to the copper opening molecular channels in the polymer. According to the team’s modelling, these channels increase the spaces between the cellulose polymer chains, which normally exist in tightly packed bundles. The expanded spacing creates what amount to “ion superhighways” through which ions can zip by relatively unimpeded, they say.
Sandwich strategy makes solid-state lithium battery last longer
As well as its high lithium-ion conductivity of 1.5 × 10–3 S/cm (a value comparable to that of ceramics), the new material is thin and flexible, and operates over a wide range of voltages of 0.2 to 4.5 V. The researchers say that their approach, which they detail in Nature, could be extended to other polymers and metal cations.




